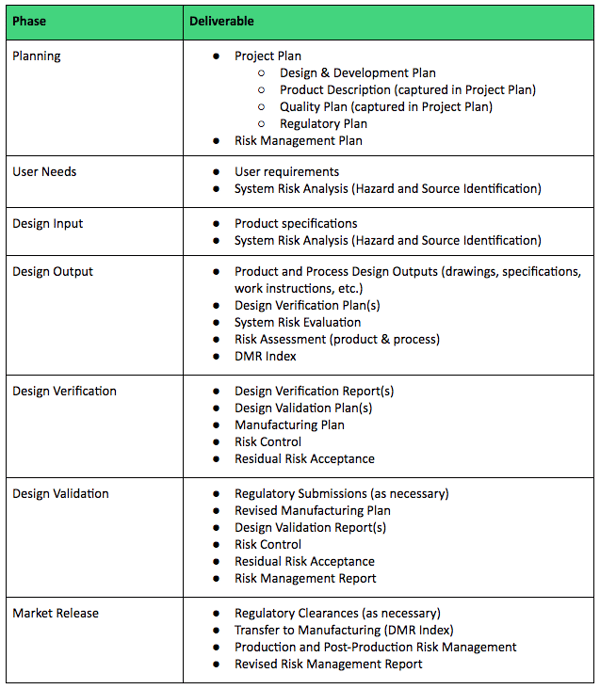
The validation protocol included with the procedure provides step by step instruction through the entire validation process. Record of software validation the record provides information about software validation results.
There are no shortcuts in this process.

Iso 13485 software validation template. Youll need to ensure that your system is working and continues to work as required. With iso 134852016medical devicesquality management systemsrequirements for regulatory purposes published and being implemented many medical device customers are experiencing some uncertainty about the effect that one of the standards key changes might have on their business. Imsxpress iso 13485 template documentation is part of imsxpress iso 13485 software.
The procedure should reference iso 134852016 and outline a risk based approach to evaluating current updated and new software that will be used in the quality system. Product information quotations and orders. Validate such software applications prior to initial use and as appropriate after changes to such software or its application.
The document is optimized for small and medium sized organizations we believe that overly complex and lengthy documents are just overkill for you. Pack of iso 13485 templates includes quality management system templates for developing policies standard operating procedures sops and work instructions for the following areas of your business. Validation of processes 756 validation of the application of computer software used in production and services provision 756 sterilization and sterile barrier systems 757.
The template documentation covers both iso 134852003 and fda qsr 21 cfr part 820 requirements under one quality system and is thus ideally suited for companies that must comply with both the us fda and international regulations. Iso 13485 software validation procedure overview the software validation procedure uses a risk based approach for conducting software validations. Requirements of iso 13485.
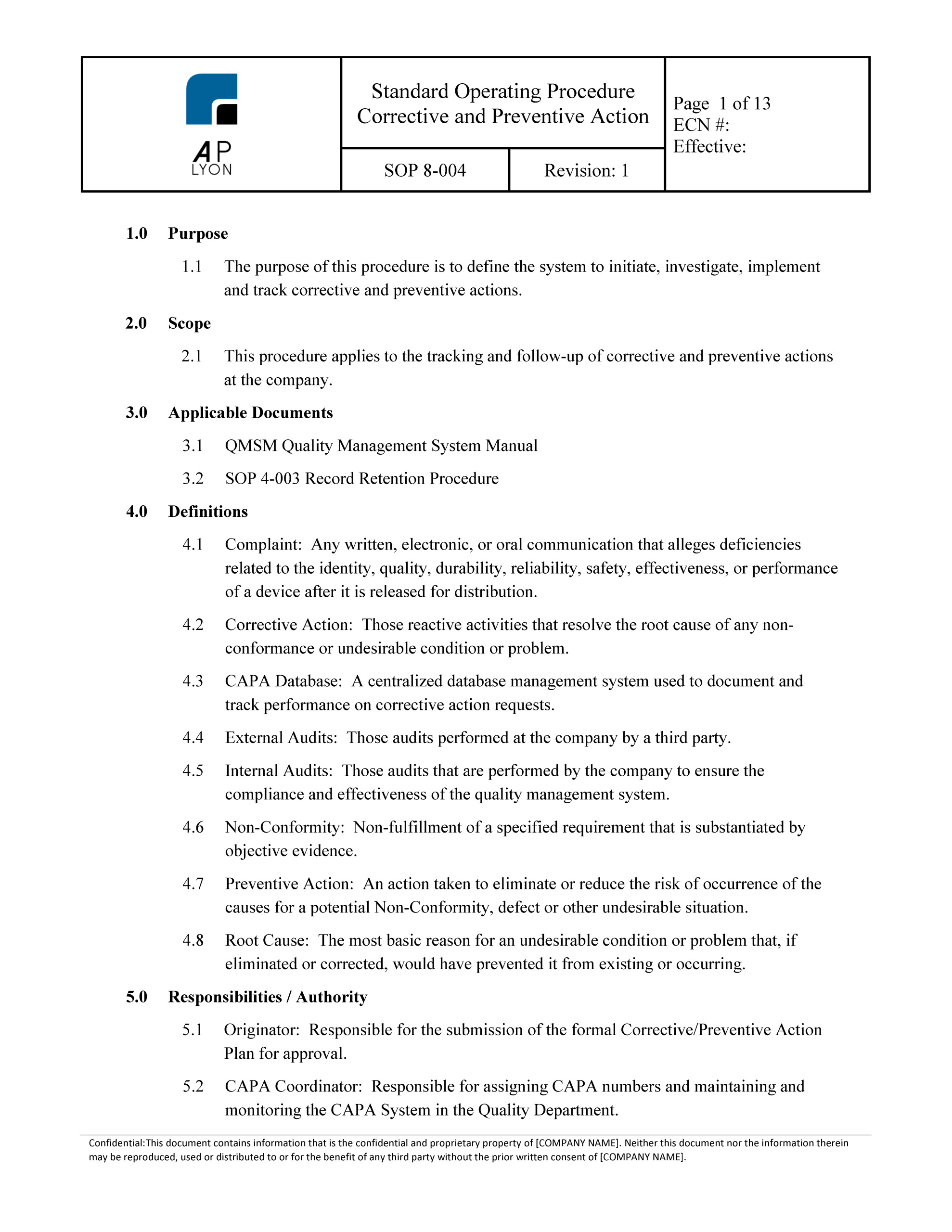
The first detail to focus on is the creation of a quality procedure or sop for the evaluation and validation of software used in the quality system. Iso 13485 software validation process when youre implementing an electronic medical device quality management system your software validation plan is of the utmost importance. Ensure that the.
Use our free iso 13485 procedure template and the list of iso 134852016 mandatory procedures to build your medical device quality system and get certified. This procedure will. 2016 and iso 17025 standards iso 13485 v 2016 416 document procedures for the validation of the application of computer software used in the quality management system.
Iso 13485 document template.










0 Response to "Iso 13485 Software Validation Template"
Post a Comment